2014
Journal Publication
PNAS
Synaptonemal complex extension from clustered telomeres mediates full-length chromosome pairing in Schmidtea mediterranea
Xiang Y, Miller DE, Ross EJ, Sánchez Alvarado A, Hawley RS
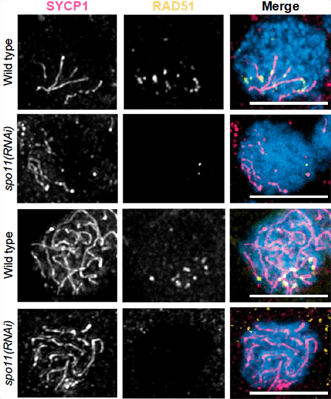
In the 1920s, József Gelei proposed that chromosome pairing in flatworms resulted from the formation of a telomere bouquet followed by the extension of synapsis from telomeres at the base of the bouquet, thus facilitating homolog pairing in a processive manner. A modern interpretation of Gelei’s model postulates that the synaptonemal complex (SC) is nucleated close to the telomeres and then extends progressively along the full length of chromosome arms. We used the easily visible meiotic chromosomes, a well-characterized genome, and RNAi in the sexual biotype of the planarian Schmidtea mediterranea to test that hypothesis. By identifying and characterizing S. mediterranea homologs of genes encoding synaptonemal complex protein 1 (SYCP1), the topoisomerase-like protein SPO11, and RAD51, a key player in homologous recombination, we confirmed that SC formation begins near the telomeres and progresses along chromosome arms during zygotene. Although distal regions pair at the time of bouquet formation, pairing of a unique interstitial locus is not observed until the formation of full-length SC at pachytene. Moreover, neither full extension of the SC nor homologous pairing is dependent on the formation of double-strand breaks. These findings validate Gelei’s speculation that full-length pairing of homologous chromosomes is mediated by the extension of the SC formed near the telomeres. S. mediterranea thus becomes the first organism described (to our knowledge) that forms a canonical telomere bouquet but does not require double-strand breaks for synapsis between homologous chromosomes. However, the initiation of SC formation at the base of the telomere bouquet, which then is followed by full-length homologous pairing in planarian spermatocytes, is not observed in other species and may not be conserved.
Address reprint requests to: Alejandro Sánchez Alvarado